Abstract
Background: At least 26 different fecal immunochemical tests (FITs) are available for use in the US. Liquid vial and card collection devices are available.
Objectives: 1) assess participant’s difficulties with and preferences for types of FITs; 2) assess whether errors in FIT collection were associated with FIT collection difficulty; 3) identify factors associated with difficulty with FIT stool collection.
Methods: Prospective individuals scheduled for a colonoscopy were invited to participate in a study comparing test characteristics of 5 FITs. A product questionnaire asked participants about ease of collection and difficulties.
Results: 2,148 participants; mean age 63 years; 63% females, 83% Whites, and 19% Hispanics. 1265 (61%) preferred use of a liquid vial versus 181 (9%) the card. 49% had no difficulty with Hemoccult ICT, and 66 to 70% had no difficulty with the liquid vials. Difficulties with Hemoccult ICT included: being messy (21%), collection window too small (19%), and getting sample on stick (8%). Difficulties with the liquid vials included difficulty probing or scraping the stool (5% to 8%) and unclear directions (3%). In a multivariable model, the perceived difficulty in FIT collection was significantly higher for Hemoccult ICT compared with OC-Auto Micro (adjusted odds ratio [AOR], 4.05), and it was significantly high for those with a FIT error (AOR, 3.90).
Conclusion: Participants strongly preferred a liquid vial compared with a card. Perceived difficulty was significantly associated with FIT errors and with FIT brand. Medical offices providing FITs should ensure that patients understand the task of FIT collection, so that errors are minimized.
- Cancer Screening
- Colorectal Cancer
- Fecal Occult Blood Test
- Fecal Immunochemical Tests
- Surveys and Questionnaires
Fecal immunochemical tests (FITs) can be a sensitive, specific, and low-cost alternative to colonoscopy.1 Annual fecal occult blood tests (FOBTs) (FIT or guaiac) are 1 of 5 colorectal cancer (CRC) screening tests recommended by the US Preventive Services Task Force.2,3 Over a lifetime, FITs completed annually are estimated to avert 25 CRC deaths per 1000 people, compared with 27 deaths per 1000 averted by colonoscopy every 10 years.3 FITs are preferred over guaiac tests for FOBTs. FITs are widely available, inexpensive, and highly cost-effective compared with colonoscopy for CRC screening.4 The effectiveness of any CRC screening test depends on the accuracy of the test characteristics and adherence by patients.5,6
Little research with extremely low sample sizes has been conducted on patient preferences regarding use of FITs. In a comparison of 3 methods for transferring stool to a dry-slide collection card in North Dakota, 24 of 47 (51%) convenience sample participants found the traditional wooden stick to be the most preferred method, compared with stool collection of toilet tissue stool smear, or direct smear from the stool to the dry-slide card.7 In another extremely small study in Oregon, 4 of 11 participants completing 6 FITs reported the Hemoccult ICT wooden stick was difficult to use and 12 participants out of 12 preferred the OC-Light FIT probe for the liquid vial sample collection method.8
There are many FITs sold in the US, with the same FIT being marketed under different names by different distribution companies. A detailed search identified 24 distinct Clinical Laboratory Improvement Amendments (CLIA)-waived FITs and 2 automated FITs.9 For this study, we used the 5 most commonly used FITs certified in pathology laboratories.9
Studies comparing difficulties of FITs used in the US have not been conducted. For this study, participants evaluated 5 FITs, while they participated in a main study comparing test characteristics of the FITs with optical colonoscopy.5 The purposes of this study were to: 1) assess participant’s difficulties with and preferences for types of FITs; 2) assess whether errors in FIT collection were associated with FIT collection difficulty; and 3) identify factors associated with difficulty with FIT stool collection.
Methods
The main prospective study invited those 50 to 85 years of age scheduled for a screening or surveillance colonoscopy to participate in a study comparing test characteristics of 5 different FIT products using colonoscopy as the reference standard.5 Institutional Review Board approval was received at each of the 3 participating sites: University of Iowa (UI) in Iowa City, Iowa; University of North Carolina (UNC) at Chapel Hill, North Carolina; and Texas Tech University Health Sciences Center (TTUHSC) at El Paso, Texas.5 Clinical trials: NCT03264898.
After a signed informed consent and health questionnaire that included demographics were received, participants were mailed or handed a package with 5 different FITs, detailed enlarged directions for each FIT, a plastic specimen container pan, a card to record the date of stool collection, a cardboard postage-paid return mailer, and a product questionnaire. All samples were returned to the UI where researchers analyzed the tests.5 On return of the FITs and completion of the colonoscopy, participants were paid $25. The main study started 9/20/2017. When we noticed participants making FIT collection errors, the product questionnaire was developed. The first subject returned the product questionnaire 12/11/2018. The study ended 11/18/2022.
Fecal Immunochemical Tests (FITs)
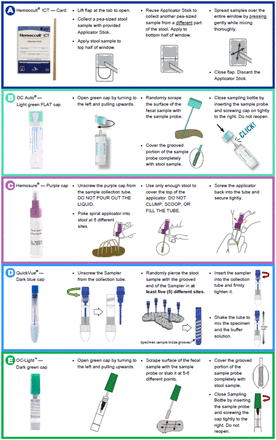
Each participant was provided 5 FITs: 4 CLIA-waived FITs, Hemoccult ICT (Beckman Coulter, Inc.), Hemosure iFOB (Hemosure, Inc.), QuickVue iFOB Test (Quidel Corporation), and OC-Light S (Eiken Chemical Company distributed by Polymedco, Inc), and a non-CLIA waived moderate complexity automated FIT, OC-Auto Micro FIT (Eiken Chemical Company distributed by Polymedco Inc). The FITs chosen were the most commonly used in pathology proficiency testing programs in the US, required to perform waived tests having a CLIA Certificate of Waiver.9 The products included 4 liquid vials with screw-on caps and 1 dry-slide card (referred to as card) (Hemoccult ICT). Patient instructions from each manufacturer were enlarged and color coded by FIT liquid vial/card color and placed on 1 large flier for ease of participant use (See Appendix A). Participant FIT collection errors (overall errors, card errors, and liquid vial errors) were tracked during the study.10
Product Questionnaire
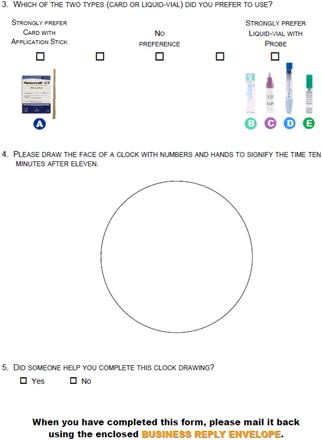
A 13-item investigator-developed product questionnaire was developed a few months into the study (See Appendix B). For each FIT used in the study, questions were asked about ease of collection and any difficulties with collection, with participants able to choose all that applied from a list of options. For data analyses on FIT preference, the 5-point scale was collapsed to 3 categories: 1) preferred card (strongly preferred and preferred, box next to strongly preferred), 2) no preference, and 3) preferred liquid vial (strongly preferred and preferred, box next to strongly preferred) (See Appendix B). Two questions to partially assess cognitive impairment instructed the participants to draw a clock depicting the time as ten minutes after eleven and to indicate if anyone helped them draw the clock. The clocks were scored by 2 methods, Watson et al. and Mendes-Santos et al.10⇓–12
Data Analysis
All data were double entered by 2 different research assistants. A comparison of all entries was conducted and those with discrepancies were reviewed and corrected. Standard descriptive statistics were used to summarize the variables and assess the distributions of responses for key variables. Participants included in our analyses returned 4 of 5 FITs and the product questionnaire. The main outcome variable for this study was the patients’ experience of how easy it was to collect the specimen with scores from 1 = “very easy” to 5 = “very difficult” for each FIT. These scores were dichotomized as follows: scores of 2, 3, 4, and 5 were categorized as having “some difficulty,” and a score of 1 was categorized as “no difficulty.” For each FIT, if a participant made any errors, that FIT was categorized as having an error. An individual could have up to 5 errors and there was no weighting by FIT.
Generalized linear mixed models, using the SAS GLIMMIX procedure were used, to examine a binary outcome of whether having some difficulty versus no difficulty collecting the stool samples were different among the 5 FITs, adjusting for demographic factors, FIT errors, and each of the clock drawing scores. The participant was specified as the random intercept in the model to account for the correlation of the scores of 5 FIT responses for each participant. The site variable was not specified as a random effect because there was not significant variance among 3 sites. Binary distribution and logit link were specified in the model. Each of the potential associated factors was tested individually with the outcome variable in the model and included FIT brand, patient’s age, sex, race, ethnicity, household income, education, FIT collection error (yes or no for each of 5 FITs) and each of the 2 clock drawing scores separately. Variables with P < .15 in these univariable predictor models were included in the multivariable analyses. Subsequently, the backward stepwise method was used to remove the nonsignificant variables 1 at a time. Age, income, and education interaction terms were not significant in the multivariable model. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
In this study, 2213 participants returned the product questionnaire. Of these, 65 were excluded because they did not return FITs or did not attempt the clock drawing or had help drawing the clock, leaving a total 2148 participants who returned either 4 (n = 8) or 5 (n = 2140) FITs. Mean age of the participants was 62.8 years; 1353 (63%) were female, 1787 (83%) White, and 403 (19%) Hispanic. 1621 (76%) participants had college education or higher, 942 (44%) had income of $80,000 or higher, 453 (21%) participants had cognitive impairment by the Mendes-Santos method and 581 (27%) by the Watson method (See Table 1).
Demographic Characteristics of Study Participants
Nearly half (49%) had no difficulty with Hemoccult ICT collection, whereas only 66% to 70% had no difficulty with the liquid vials. Difficulties with Hemoccult ICT included: 450 (21%) felt it was too messy, 401 (19%) reported the collection window was too small, 306 (14%) had difficulty getting the sample in the collection window, 172 (8%) had difficulty getting the sample on the collection stick, and 73 (3%) reported the instructions were unclear (See Table 2).
FIT Collection Difficulties (n = 2,148)
Difficulty probing the stool multiple times was an issue for about 5% of the subjects for Hemosure iFOB, OC-Light S, and QuickVue iFOB liquid vials and difficulty scraping the stool was a problem for 8% of the subjects for the OC-Auto Micro liquid vial. Across the 4 liquid vials, about 3% of the participants said it was messy to collect the specimen using a probe. For each type of liquid vial, fewer than 1% of participants reported liquid spilling from the liquid vial itself and about 1% thought the instructions were unclear (See Table 3).
FIT Collection Difficulty and FIT Errors**
Removing the cap or removing the correct cap was an issue for some individuals with the liquid vials. For OC-Light, 165 (8%) of the subjects had difficulty getting the cap off. For Hemosure iFOB, 66 (3%) had difficulty getting the cap off and notably, that liquid vial has 2 caps at the same end of the liquid vial. For QuickVue iFOB, 137 (6%) of the participants did not know which cap to open, as there was a cap on each end, even though the directions are clear (See Table 2, Appendices A and B). For OC-Auto Micro 53 (2.5%) participants had trouble removing the cap.
Participants preferred use of the liquid vials compared with the card 1265 (61%) versus 181 (9%), with 633 (30%) reporting no preference. For each of the 5 FITs, those participants who perceived “some difficulty” had significantly more errors than those who perceived “no difficulty.” For example, for Hemoccult ICT, 2.4% of participants had “no difficulty” made an error compared with 4.2% of participants who perceived “some difficulty” (P = .027). Overall FIT errors ranged from 0.4% to 3.2% with most errors noted on the card (See Table 3). All OC-Auto Micro samples were analyzed in the main pathology lab, regardless of whether there appeared to be stool in the liquid vial; thus no errors were able to be calculated for this FIT.
Univariable results indicated participants had statistically significantly more difficulty collecting the Hemoccult ICT stool sample than the other FITs. There were significant differences in perceived FIT difficulty by age, ethnicity, education, income, and FIT errors. Those with FIT errors had significantly more difficulty collecting their samples. Participants aged younger than 65 years, those with a high school education, those with a household income less than $40,000, and those who were Hispanic reported significantly less difficulty collecting the stool sample than their comparison groups (See Table 4).
Factors Associated with Having Some Difficulty versus No Difficulty in Collection of FIT Sample (n = 2,148)*
In the multivariable model, FIT collection for Hemoccult ICT was significantly more difficult than OC-Auto Micro (Adjusted odds ration [AOR], 4.05; 95% CI, 3.34-4.85); and Hemosure iFOB was significantly less difficult than OC-Auto Micro (AOR, 0.72; 95% CI, 0.59-0.88), after controlling age, education, household income, and FIT errors. Collection difficulties were not statistically significantly different for OC-Light S and QuickVue iFOB compared with OC-Auto Micro. FIT errors were highly associated with perceived difficulty in FIT collection (AOR, 3.90; 95% CI, 2.46-6.17) Those aged younger than 65 years compared with those 65 to 75 years (AOR, 0.65; 95% CI, 0.51-0.83), those with high school education compared with college or higher (AOR, 0.68; 95% CI, 0.49-0.93), and those having a household income less than $40,000 compared with income of $80,000 or more (AOR, 0.60; 95% CI, 0.44-0.81) perceived significantly less difficulty in FIT collection. (See Table 4).
Discussion
This is a large multi-site study that describes patient difficulties with 5 commonly used FITs in the US, whereas earlier studies assessed patient preferences for FITs.7,8 A liquid vial with a probe attached to the cap is preferred to the card with wooden stick for collection.
Difficulties with the card included participants feeling the window was too small, being unsure where to place the stool, and feeling that collection was messy. Difficulties with the liquid vials included the cap being on too tight or confusion over which cap to open when there were 2 caps. Three of the 4 liquid vials had 2 caps. OC-Auto Micro had 1 cap and a foil seal on the opposite end. Hemosure iFOB and OC-Light S had the color of the cap to be removed clearly shown in the directions, but some patients removed the wrong cap. Those were slightly minor problems for the liquid vials, and one may wonder if the person opening the liquid vial was frail or had other physical or cognitive issues.
Another minor problem was the directions were perceived as unclear by some participants for each of the 5 FITs. The directions for this study were copied verbatim and enlarged from the manufacturer’s directions to more closely mimic what patients might receive in practice. In another small study, wordless directions were developed for FIT collection and preferred by patients in qualitative analysis.13 Future studies might address whether having a nursing or medical assistant provide verbal instructions, in addition to the written and pictorial directions might improve patient FIT collection.
Four of the 5 FITs had some errors noted on receipt. Although, the overall FIT error rate was low at 3.2% or less, those who perceived difficulty in FIT collection had more errors for 4 of the 5 FITs. Since the OC-Auto Micro was sent to our main pathology laboratory on arrival and it can be analyzed with no stool, no errors were calculated for that FIT.
Fecal blood testing was developed in 1864 with the use of gum guaiac. The dry-slide guaiac card was developed in the 1960s, followed by the development of immunochemical tests in the late 1970s.14⇓⇓⇓–18 The main collection device became the dry-slide card and remained so until FITs were available with most manufacturers using a liquid vial for collection.19 FITs are superior to guaiac tests specifically in the detection of advanced neoplasia and colorectal cancer in average-risk individuals.20 Only a few participants had some difficulty with FIT collection, with Hemoccult ICT being the primary FIT with the most difficulty, as compared with liquid vial FIT tests. Participants in this study had the least difficulty using the liquid vials, similar to other much smaller studies, where the liquid vial was preferred.8,21 Although most participants preferred the liquid vial, a sizable percentage, 30%, were neutral on the issue. The accuracy of individual FIT tests should be a major consideration in colorectal cancer screening, but there is relatively little data on this.6,22,23 In 2024, cost for a card and analysis is approximately $14 compared with a liquid vial at $32 (Fischer Scientific online catalog at https://www.fishersci.com). Different FITs have varying sensitivity and specificity for detecting hemoglobin, thus practitioners should note this when choosing a FIT.5,6,22,23
Prior studies comparing FIT preferences did not include as many demographic characteristics as this study. A study comparing a wooden stick stool collection against a smear or tissue collection found no difference in preference by sex.7 Our study also found no differences by sex for difficulty collecting the stool specimens. Other research showed that participants who were younger, female, had higher household income, and had previously used a FIT were significantly more satisfied with using a FIT liquid vial compared with a plain plastic container.24 Similar to the Shin study,24 our younger participants had less difficulty collecting the FIT sample. But, in another study comparing guaiac and FIT stool collection, persons over 60 years compared with persons 50 to 60 years of age perceived the collection easy to perform for both tests.21
In the multivariable model, among the FITs, Hemoccult ICT was statistically significantly more difficult to complete and Hemosure iFOB less difficult to complete than OC-Auto Micro. FIT collection errors were highly associated with FIT collection difficulty. Factors statistically significantly associated with less difficulty collecting stool specimen were age < 65 years compared with those 65–75 years, those with high school education compared with college or higher education, and having household income of less than $40,000 compared with those with $80,000 or more.
Two results in the generalized linear mixed model seemed counterintuitive: lower income and lower educational level were associated with having a perception of less difficulty with FIT collection. Our study included participant level of education, which had not been collected in other studies assessing preferences of FITs.7,8,21,24,25 In our prior publication regarding the errors in FIT collection based on a subset of this study sample, those with an eighth grade education or less compared with high school or higher education, being female, and an abnormal clock drawing had a higher odds of a FIT collection error.10 Stool sample collection requires adequate physical performance and cognitive function to be completed correctly. Directions must be followed to be successful. Our findings indicated some subjects had cognitive impairment by the clock-drawing test, but they did not perceive collecting the specimen as difficult and clock drawing accuracy did not enter the multivariable model. Some subjects may not realize they collected the specimen incorrectly. Perception of less difficulty does not mean the sample was collected correctly.
Strengths and Limitations
This was a large, diverse study of individuals from the US which assessed patient’s difficulties with FIT collection with detailed documentation of FIT collection errors. FIT directions per the manufacturer were enlarged and provided word-for-word onto a single page in colors that correspond to the manufacturer’s directions. In addition, to encourage participation by Spanish-speaking individuals, the directions and study materials were translated into Spanish. We were able to control for several demographic factors. We excluded those who did not complete or who had help with the clock drawing. This study is likely to be generalizable to the US and other developed countries due to its large and diverse sample size.
A limitation of the study was that each participant was expected to complete 5 FITs, whereas in practice, a patient would only be completing 1 FIT. There may be some response bias in that those who could not read English or Spanish may have chosen not to participate. Manufacturer’s direction sheets were not provided to the participants but instead were copied all on 1 page. The ease of collection for each FIT was collected using a 5-point Likert scale, which was collapsed to a dichotomous variable, as the distribution of the 5-point scales were not normal. Individuals who perceive that they had no difficulty may not have collected their sample correctly.
Conclusion
Nearly two-thirds of participants preferred the liquid vial compared with the card for FIT collection. Although the FIT collection error rate was below 3%, those who perceived more difficulty had significantly more errors in FIT collection. The multivariable model demonstrated that the Hemoccult ICT was substantially more difficult to collect than OC-Auto Micro and Hemosure iFOB was less difficult to collect than OC-Auto Micro. Medical offices providing FITs should ensure that patients understand the task of FIT collection, so that errors are minimized.
Appendix A. Participant Instructions for Collecting and Returning Five FITs.

Appendix B

Notes
This article was externally peer reviewed.
Funding: Research reported in this publication was supported by the National Institutes of Health, National Cancer Institute R01 CA215034 (BT Levy, PI), the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002537 (P Winokur, PI), and the National Institutes of Health, National Cancer Institute P30 CA086862 (G Weiner, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: The authors have no conflicts of interest.
To see this article online, please go to: http://jabfm.org/content/37/6/1014.full.
- Received for publication December 13, 2023.
- Revision received May 2, 2024.
- Accepted for publication May 20, 2024.






