Abstract
Background: The Papanicolaou (Papanicolaou) test is an effective and widely used cervical cancer screening procedure. Recommendations for cervical cancer screening do not incorporate patients’ gender identities nor gender-affirming hormone statuses in determining screening surveillance intervals and interpreting test results. This study assessed the association between testosterone and rates of abnormal Papanicolaou specimens and specimen adequacy by comparing testosterone-associated Papanicolaou specimens and nontestosterone Papanicolaou specimens among transgender and gender-diverse (TGD) patients.
Methods: Retrospective electronic health record chart review of 211 TGD patients with cervixes, contributing 298 unique Papanicolaou tests, with a visit to a primary care clinic between 2012 and 2019. Primary outcomes included specimen quality (transformation zone [TZ] present, TZ absent, atrophic specimen, scant cellularity), presence of inflammation (yes/no), and cytology results (normal, abnormal, inadequate specimen). χ2 and t test were used to compare results between testosterone-associated Papanicolaou specimens (TAPS) and nontestosterone specimens (NTS).
Results: A higher proportion of TAPS had missing TZ, showed atrophy, or had scant cellularity than NTPS (58.8% vs 33.5%; P < .001). In addition, a higher proportion of TAPS showed inflammation (16.0% vs 3.4%; P < .001). There was no significant difference in the proportion of abnormal results (ie, cytologic features concerning for dysplasia) between the TAPS and NTPS groups.
Conclusions: Findings confirmed an association between testosterone usage and cytology specimen adequacy and quality. No association was found between testosterone usage and rates of abnormal results within our TGD population. More research is needed to disentangle the effect of missing TZ on risk of future cervical dysplasia among this younger population.
- Cancer Screening
- Cervical Cancer
- Gender Identity
- Hormone Replacement Therapy
- Papanicolaou Test
- Primary Health Care
- Retrospective Studies
- Testosterone
Introduction
The Cervicovaginal Papanicolaou (Papanicolaou) test is an effective and widely used screening procedure for the detection of cervical cancer. Current guidelines recommend the test for patients with cervixes, regardless of their gender identities, who are between the ages of 21 and 65.1 These recommendations do not incorporate patients’ gender identities nor gender-affirming hormone statuses in determining screening surveillance intervals and interpreting test results, despite research showing the impacts of testosterone therapy on the quality, adequacy, and appearance of cervical cytology specimens.2⇓⇓–5 There are, however, mixed conclusions regarding testosterone’s impact on cervical cytology. For example, studies to date have produced mixed data on the association between testosterone usage and abnormal (ie, dysplastic) Papanicolaou specimens.3,6,7 In addition, there is uncertainty regarding the effects of testosterone on specimen adequacy. Indeed, reported rates of inadequate Papanicolaou specimens among transgender patients range from 6% to 13%.4,7,8
Given the conflicting evidence, this study utilized chart review to assess the association between testosterone and rates of abnormal Papanicolaou specimens and specimen adequacy by comparing testosterone-associated Papanicolaou specimens and nontestosterone Papanicolaou specimens among TGD patients. We hypothesized that testosterone usage will be associated with higher rates of abnormal Papanicolaou specimens and higher rates of inadequate Papanicolaou specimens.
Methods
We conducted a retrospective electronic health record chart review of TGD patients with cervixes at an academic health center. Patients included in our study had at least one visit to a primary care clinic between 2012 and 2019. We identified TGD patients with cervixes using demographic data (sex at birth, legal sex, gender identity), organ inventory, and gender dysphoria diagnostic codes. For patients who were identified, 2 researchers independently reviewed these individuals’ charts and coded cytology results of Papanicolaou tests performed between 01/01/2012 and 12/31/2022 as well as patients’ testosterone statuses before their Papanicolaou tests. Samples from patients who were under 21 at the time of their Papanicolaou tests were excluded. Data concordance between the coders was 95% and discrepancies were reconciled by the senior author and included in the analysis. Primary outcomes included specimen quality (transformation zone [TZ] present, TZ absent, atrophic specimen, scant cellularity), presence of inflammation (yes/no), and cytology results (normal, abnormal, inadequate specimen). Of note, atrophic or scant specimens implicitly lack indication as to whether the transformation zone was sampled. Normal results indicated specimen was negative for intraepithelial lesion or malignancy. We considered patients to be on testosterone if they began treatment at least 6 months before their Papanicolaou tests to ensure results reflected their estrogen-suppressed cervical environments.8,9 We compared outcomes between testosterone-associated Papanicolaou specimens (TAPS) and nontestosterone Papanicolaou specimens (NTPS) using the Pearson χ2 test. t test was used to compare the mean age. If a TGD patient had multiple Papanicolaou tests and started testosterone between those tests, they could contribute to both the TAPS and NTPS groups. The institutional review board approved this study.
Results
We identified 211 TGD patients who contributed 298 unique Papanicolaou tests. There was no difference in the mean age between the TAPS and NTPS groups. The mean number of Paps per patient was 1.7 (standard deviation 0.9).
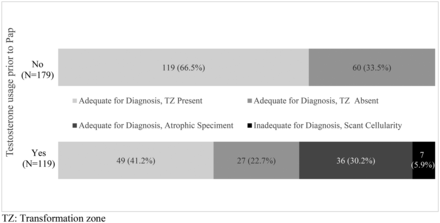
Results (Table 1) indicate that a higher proportion of TAPS had missing TZ, showed atrophy, or had scant cellularity than NTPS (58.8% vs 33.5%; P < .001). Among TAPS, 5.9% had scant cellularity, 30.2% had atrophic characteristics with no indication of TZ presence or absence, and 22.7% had missing TZs (Figure 1). Among NTPS, 33.5% had missing TZs; none had scant cellularity or showed atrophy. In addition, a higher proportion of TAPS showed inflammation (16.0% vs 3.4%; P < .001). When inadequate specimens were excluded, there was no significant difference in the proportion of abnormal results between the TAPS and NTPS groups.
Papanicolaou quality by testosterone therapy among 211 transgender and gender-diverse patients.
Pap Results by Testosterone Therapy among 211 Transgender and Gender-Diverse Patients
Discussion
To date, there is no previous study comparing cervicovaginal cytology between TGD patients’ according to their testosterone hormone therapy statuses; all previous studies used non-(TGD)-patients’ Papanicolaou specimens as comparison groups. Our study design helped control for gender identity-related factors when assessing testosterone’s effects on Papanicolaou results. Overall, the findings were consistent with studies that did not demonstrate an association between testosterone usage and abnormal specimens. It should be noted, however, that abnormal Papanicolaou rates in TAPS and NTPS groups (8.9%) were higher than that of the general population, which is about 3.8-5%.10,11 Future research should investigate whether there are gender-specific, nontestosterone-related factors that contribute to increased rates of abnormal Papanicolaou results. Next, while our results were consistent with studies showing a positive, association between testosterone usage and specimen adequacy, we observed a smaller rate (6%) than other studies, suggesting that factors other than testosterone usage may have measurable effects on specimen adequacy rates. For example, clinicians with less cultural competence in performing pelvic exams and Paps on TGD patients may inadvertently deviate from standard Papanicolaou specimen collection practices.12 Future research should elucidate methods to mitigate any modifiable factors, such as making trauma-informed care more widespread to helping clinicians feel more confident in performing Papanicolaou tests on TGD patients.12
The results also showed a positive association between testosterone usage and rates of inflammation in the specimens. This may be related to testosterone’s effects on TGD patients’ cervical environments and sexual behaviors. Testosterone leads to vaginal atrophy and decreased lubrication, so TGD patients on testosterone are more likely to have dry cervical environments, predisposing them to trauma leading to inflammation.13,14 This is exacerbated by increased rates of penetrative sexual activities observed among TGD patients on testosterone.15
Our findings also showed that both TAPS and NTPS groups had considerably higher rates of specimens lacking transformation zones than those in the general population, which is between 11.7% and 16.0%.16 It is important to note that the observed rate of missing TZs among the TAPS group is an underestimate as pathology reports of specimens with atrophic and scant cellularity characteristics are nonspecific about the presence of TZs. Nonetheless, the high rate of missing TZs among nontestosterone Papanicolaou specimens (33.5%) warrants attention.
The transformation zone is the site of most intraepithelial neoplasia, so sampling of the TZ is of paramount importance. Current studies indicate that specimens lacking evidence of TZs are not associated with an increased risk of future cervical dysplasia.1,16,17 The American Society for Colposcopy and Cervical Pathology’s guidelines for managing absent TZ specimens were constructed based on these findings.1 The existing literature, however, represents Papanicolaou data from a majority nontransgender cohort, so they may not be representative of trends within TGD patients. The high TZ missing rates in our study—regardless of TGD patients’ testosterone usage statuses—necessitates follow-up to investigate whether rates are similarly high for TGD patients in other health care settings. If so, it would be beneficial to assess whether an association exists between missing TZ and the future risk of cervical dysplasia among TGD patients. Since there are currently no studies that have examined this association, greater caution might be warranted in scenarios where these patients’ TZs are missing from otherwise normal-appearing specimens. This is especially important for younger TGD individuals – as HPV and cervical cancer rates are higher in all patients with cervixes who are younger than 35.20
Finally, since our study was the first to evaluate the effects of testosterone on Papanicolaou specimens using a TGD-only cohort, future studies should assess whether our findings are reproducible in other health care settings.
Limitations
Our results must be considered in the context of the study’s limitations. Our study’s patients came from one academic health center, so results may differ at other health care systems. In addition, since we did not collect information on the clinicians performing the Papanicolaou tests, we were not able to control for specimen collection idiosyncrasies between clinicians. Therefore, results may be different if we compare specimens from the same clinician. Because the practice of HPV cotesting was not routinely conducted during the time frame of our study, we were unable to determine the HPV status within our population. Future studies are needed to fill this gap. As our sample is restricted to TGD population within one health system, future studies including multiple clinics and comparison groups within clinics such as non-TGD are warranted to control for variations in collections and confirm these results.
Conclusion
Our study found no association between testosterone usage and rates of abnormal Papanicolaou specimens. We observed a small association between testosterone usage and specimen inadequacy rates. Finally, we found higher rates of cytologic specimens lacking evidence of the transformation zone having been sampled among our study population, regardless of our patients’ testosterone statuses. More research in other health care settings is needed to confirm our findings. We also call for more investigation into nontestosterone-related factors that may be affecting TGD patients’ Papanicolaou specimens, as results from these studies would benefit all individuals with cervixes.
Notes
This article was externally peer reviewed.
Funding: This work was supported by the National Cancer Institute (NCI) of the National Institutes of Health under Award Number P50CA244289. This program was launched by NCI as part of the Cancer Moonshot. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: The authors declare they have no competing interests.
To see this article online, please go to: http://jabfm.org/content/37/6/1009.full.
- Received for publication December 13, 2023.
- Revision received May 2, 2024.
- Accepted for publication May 13, 2024.