Abstract
Background: Prescription biosimilars are highly similar to and have no clinically meaningful differences from existing FDA-approved reference products. Despite increased availability in the marketplace, consumers and clinician lack awareness of these products. Our study experimentally tested understanding of and preference for brief descriptions of biosimilars in the form of disclosure statements in a fictitious prescription drug advertisement.
Methods: Consumers (n = 379) and HCPs (n = 368) viewed a mock advertisement and responded to an online survey. Study participants were randomized to 1 of 7 biosimilar disclosure definitions or a control. Disclosure conditions varied with regard to 1) identifying the product as a biosimilar; 2) information provided in the definition; and 3) naming the reference product. We tested the effects of disclosure conditions on comprehension, perceptions, attitudes, intentions, and preferences.
Results: Overall, comprehension of information in the biosimilar disclosure was less than optimal (48.5%-62.0% and 68.4%-88.4% for consumers and HCPs, respectively), even when provided with a definition. Perceptions of benefit, comparative efficacy, and safety were neutral. Content of the biosimilar definition generally did not influence outcomes, except that HCPs reported more positive attitudes toward the biosimilar and stronger intentions to prescribe when provided with expanded definitions. Both groups preferred the expanded definitions, and HCPs preferred seeing a named reference product. HCPs generally agreed with a statement that biosimilars could be used “interchangeably” with the reference product.
Conclusions: Our findings signal some knowledge gaps and uncertainty regarding biosimilars among consumers and HCPs. Further education is warranted around these products, and communications for both groups require careful testing to ensure that the information is understood and does not result in a negative perception of the product.
- Advertising
- Biosimilar Pharmaceuticals
- Comprehension
- Direct-to-Consumer Advertising
- Disclosure
- Health Communication
- Patient Education
- Perception
- Prescription Drugs
- Public Health
- Quantitative Research
Introduction
A prescription biosimilar is “highly similar to, and has no clinically meaningful differences in terms of safety, purity, and potency (safety and effectiveness) from an existing FDA-approved biologic, called a reference product.”1 Biosimilars are evaluated for FDA approval by demonstrating biosimilarity between the proposed biosimilar and its reference product, allowing for an abbreviated yet rigorous approval pathway that may not need to involve lengthy clinical trials. Approved biosimilars are available for the treatment of many medical conditions, including, for example, chronic skin diseases, bowel diseases, arthritis, kidney conditions, and cancer.2,3 The availability of biosimilars in the marketplace has increased in recent years, expanding access to lifesaving medications, allowing for more treatment options, and potentially lowering health care costs.4
Despite the increased availability of biosimilars and possible benefits, both health care consumers and prescribers lack familiarity with and have limited understanding of biosimilars. A 2017 survey of 1200 US specialty physicians who represented areas of high biologics prescribing revealed knowledge gaps around biosimilar-related definitions, perceptions of safety, and efficacy as compared with the reference product.5 There was also some misunderstanding around the term “interchangeability.” Other studies of various medical specialties have found similar knowledge gaps.6–9 Research has also signaled some hesitancy in prescribing biosimilars over the reference product because of perceptions of lower efficacy and safety.9,10 Studies with health care consumers suggest that awareness of these products is generally low,11 and concerns that biosimilars may yield more side effects or that switching from the biologic to the biosimilar may cause adverse reactions are prevalent.11–13 Providing information on the efficacy and risk profile of biosimilars relative to their referent medication may be a valuable approach to correcting some of the misconceptions or increasing understanding of biosimilars.
Both health care providers (HCPs) and adult health care consumers are widely exposed to prescription drug promotions through such sources as printed media, television, internet, and social media. In 2020, the Food and Drug Administration (FDA) published draft guidance on promotional labeling and advertising for prescription biosimilars, including recommendations for presenting this complex information through use of disclosure statements.14 Disclosures in prescription drug promotion can help draw attention to additional detail or context about the information. When noticed and understood, disclosures can enhance understanding, help correct misperceptions, or clarify areas of uncertainty.15–19
The goal of the current study was to explore how to communicate information about biosimilars in prescription drug promotions in a manner that is understood, addresses misconceptions, and does not adversely influence perceptions and attitudes around these products. To investigate this aim, we tested a series of brief statements about biosimilars that appeared as disclosures on a fictitious prescription drug promotional website. We examined the effect of: (1) identifying the product as a biosimilar; (2) varying the biosimilars definition; and (3) naming the reference product. We sought to know how the disclosures are understood and perceived by both consumers and HCPs and their preferences among the different disclosures.
Thus, this study contributes to existing research by offering perspectives on language to effectively define biosimilars in prescription drug promotions to clarify potential misconceptions and to ultimately inform and educate consumers and HCPs about biosimilars in a neutral manner that neither positively or negatively sways their perceptions and attitude toward the drug.
Methods
Participants
Participants were recruited from an online US consumer and HCP panel. Inclusion criteria for consumers included aged 18 years or older, ability to read and speak in English. HCPs were primary care providers (PCPs) who had prescribing authority: physicians, nurse practitioners, and physician assistants. Exclusion criteria included a pharmaceutical or marketing background or previous training in health care for the consumer sample and self-reporting seeing patients less than 50% of the time for the HCP sample. Study participants did not need to be diagnosed with or prescribe for the medical condition presented in the survey (rheumatoid arthritis). Study participation on a mobile device was not permitted and required a tablet or computer to clearly view the stimuli. This research met the criteria for exemption from the reviewing Institutional Review Board.
Study Design
We created an advertisement for a fictitious biosimilar called Kesterin, indicated for the treatment of rheumatoid arthritis (see examples in Figure 1). The advertisement was presented on a mock branded website, where the content was purposefully blurred so that only the biosimilar disclosures were visible. Our experiment manipulated the content for the biosimilar disclosures across study conditions such that they (1) identified the product as a biosimilar (ie, “Kesterin is a biosimilar”) for all study conditions except the control; (2) varied the biosimilar definitions in an additive manner, where each iteration of the definition built on the previous one by including additional information; and (3) either named the reference product Mytrozen or made mention to the reference product more generally (eg, “existing FDA-approved reference product”). The resulting design included seven experimental conditions and one control condition that did not include a disclosure (ie, a blank advertisement with the branding only). Disclosure language for the consumer and HCP groups was tailored to the samples. The consumer disclosures used lay language and the HCP disclosures used more medical language. For example, “original” replaced “reference” in the consumer questionnaire when referring to the product that came first.
Example consumer (top) and health care providers (HCPs) (bottom) biosimilar advertisement disclosure.
Participants were randomly assigned to an experimental condition using permuted block randomization in blocks of 8 (each block included each experimental condition once). We created separate randomization schemes for consumers and HCPs. Before implementing the main study, we conducted cognitive testing of survey items followed by a pretest (n = 216 each population group) to assess for clarity and potentially problematic items.
Outcome Measures
Three questions assessed participants’ gist comprehension of the biosimilar definition that they received (per their random experimental assignment). Comprehension concepts assessed whether participants understood that a biosimilar “provides the same treatment benefits as the biologic,” “is made from the same types of sources as the biologic,” and “is given the same way and has the same strength and dosage as the biologic.” Response options were true, false and do not know.
Four questions assessed perceptions of biosimilar benefits, adapted from previously validated items.20 On a scale ranging from 1 to 6, we asked participants the likelihood that Kesterin would improve rheumatoid arthritis symptoms (not likely at all [1] to extremely likely [6]), the magnitude of improvement (no improvement [1] to substantial improvement [6]), whether Kesterin is more effective than other prescription drugs that treat the same medical condition (strongly disagree [1] to strongly agree [6]), and whether Kesterin is more effective than Mytrozen (strongly disagree [1] to strongly agree [6]).
Four questions assessed perceptions of biosimilar risk, also adapted from previously validated items.20 On a scale ranging from 1 to 6, we asked participants the likelihood that people would experience at least one side effect if they took Kesterin (not likely at all [1] to extremely likely [6]), seriousness of side effects (not at all serious [1] to extremely serious [6]), whether Kesterin is safer than other prescription drugs that treat the same medical condition (strongly disagree [1] to strongly agree [6]), and whether Kesterin is safer than Mytrozen (strongly disagree [1] to strongly agree [6]).
Attitudes toward Kesterin were assessed on a semantic differential scale ranging from 1 to 6 where participants indicated whether they thought the biosimilar was a bad product (1) to a good product (6); harmful (1) to helpful (6); and not useful (1) to useful (6). For each audience (consumers and HCPs), one question assessed participants’ hypothetical intentions to use (consumer) or prescribe (HCP) the biosimilar for rheumatoid arthritis, from strongly disagree (1) to strongly agree (6). In addition, HCPs were asked the extent to which they agreed that the biosimilar and reference product “can be used interchangeably” from strongly disagree (1) to strongly agree (6).
To assess preferences for the biosimilar disclosures, we showed each participant their assigned disclosure versus one of the other disclosures and asked them to indicate their preference between the two. The presentation order of the two disclosures was randomized to control for potential order effects.
In addition to these outcomes, we assessed certain background and medical characteristics including HCPs’ familiarity with biosimilars, whether consumers had ever been diagnosed or treated for rheumatoid arthritis, and a measure of whether they reported needing help reading or understanding health information as a proxy for health literacy.
Data Analysis
Our study was powered to detect small to moderate effect sizes (based on Cohen’s threshold f < . 25 for medium effect size).21 We used Analysis of Variance (ANOVA) to assess the effect of the experimental condition on continuous outcomes and logistic regressions for categorical outcomes. We first tested for an overall effect of experimental manipulation. If statistically significant (P-value <0.05), we then conducted a series of planned contrasts using a Bonferroni adjusted P-value. Specifically, we compared the control condition (no disclosure) to all other disclosure conditions combined to test for the effect of identifying the product as a biosimilar (P-value <0.05). To test for effects of the biosimilar definition, we compared the condition that identified the drug as a biosimilar only (Kesterin is a biosimilar) to the other definitions that added information on safety and efficacy; that biosimilars are made from the same types of sources as reference products; and have the same dosage and administration. We repeated these same analyses comparing the additive biosimilar definitions to the no disclosure control (adjusted P = .025 to account for two comparisons). To examine the effect of naming the reference product, we compared the disclosure conditions that named Mytrozen to those that used the language “existing FDA-approved reference product” (P-value <0.05). Covariates were included for individual models, as determined through tests of correlation. They included age, health literacy (consumers), previous knowledge about biosimilars, and years in medical practice (HCPs).
To assess preferences for each of the seven biosimilar disclosures, we created a composite variable to reflect proportions of pairings where a biosimilar disclosure was the preferred option. To control for potential exposure effects, we examined this variable separately according to whether the participants were or were not assigned to the biosimilar disclosure condition. Analyses were conducted using SPSS version 25.
Results
The study findings are presented separately for the consumer and HCP groups.
Study Sample Characteristics
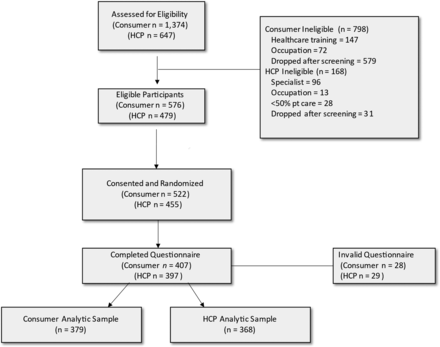
For consumers, 1374 respondents were assessed for eligibility; 576 were eligible and 379 were used in the analysis (see Figure 2). For HCPs, 647 respondents were assessed for eligibility; 479 were eligible and 368 were used in the analysis. Table 1 summarizes the demographic characteristics of the sample. One-half or more of consumers were female (53.3%), non-Hispanic White (68.9%), and had less than a college degree (66.2%). Mean age was 53 years. About 10% of consumers reported a history of rheumatoid arthritis and about 8% had ever taken prescription drugs for this condition. Only 14.5% of consumers reported being aware of biosimilars. Over half of HCPs were male (67.7%) and non-Hispanic White (57.1%), and the mean age was 51 years. HCPs practiced medicine for an average of 19 years and wrote an average of 130 prescriptions per week. About 72.6% of HCPs reported being aware of biosimilars.
Recruitment flow diagram. Abbreviation: HCP, Health care provider.
Participant Characteristics
Comprehension
Accurate comprehension for the three individual survey items ranged from 48.5% to 62.0% for consumers and 68.4% to 88.4% for HCPs (Table 2). Comprehension was lowest for the item “Kesterin has the same safety and efficacy as the original biologic” for both groups.
Comprehension of Information by Biosimilar Disclosure Characteristic
For consumers, there was no significant main effect of biosimilar definition or naming the reference product. Omnibus tests for each of the three comprehension items were Kesterin has the same safety and efficacy as the original biologic/Mytrozen (Wald X2 = 4.68, P-value = 0.457); Kesterin is made from the same types of sources as the original biologic/Mytrozen (Wald X2 = 6.49, P-value = 0.090); and Kesterin had the same strength and dosage as the original biologic/Mytrozen (Wald X2 = 0.01, P-value = 0.987). For HCPs, there also were no significant main effects of biosimilar definition or naming the reference product. Omnibus tests for each of the three comprehension items were Kesterin is highly similar to and has no clinically meaningful differences from an existing FDA-approved reference product/Mytrozen (Wald X2 = 6.28, P-value = 0.280); Kesterin is made from the same types of sources as the FDA-approved reference product/Mytrozen (Wald X2 = 3.06, P-value = 0.382); and Kesterin has the same route of administration, dosage form, and strength as an existing FDA-approved reference product/Mytrozen (Wald X2 = 0.75, P-value = 0.388). Because participants in the control condition did not receive the comprehension items, we did not conduct comparisons with this group.
Perceptions of Biosimilar Benefit and Risk of Side Effect
Table 3 shows mean scores for the benefit, comparative efficacy, and risk measures by biosimilar definition characteristic. For consumers, omnibus tests yielded no significant effect of biosimilar identification, biosimilar definition, or naming the reference product on any of the benefit and risk measures. Omnibus test results on these outcomes were perceived benefit magnitude (controlling for biosimilar awareness and health literacy [F7,369 = 1.35, P-value = 0.227]), benefit likelihood (controlling for age and health literacy [F7,368 = 1.56, P-value = 0.146]), perceived comparative efficacy relative to other prescription drugs (controlling for biosimilar awareness and health literacy [F7,369 = 0.81, P-value = 0.583]) and relative to Mytrozen (controlling for age and health literacy [F7,369 = 0.57, P-value = 0.565]), perceived side effect severity (controlling for health literacy [F7,370 = 1.20, P-value = 0.303]) and likelihood of side effect (controlling for health literacy [F7,370 = 0.69, P-value = 0.678]), and perceived comparative risk relative to other prescription drugs (controlling for age and health literacy [F2,135 = 1.18, P-value = 0.314]) and relative to Mytrozen (controlling for age and health literacy [F2,135 = 0.60, P-value = 0.550]).
Consumer Perceptions, Attitudes, and Intentions by Biosimilar Disclosure Characteristics
For HCPs, omnibus tests also indicated no significant effect of biosimilar identification, biosimilar definition, or the named reference product on outcomes. Test results on these outcomes were perceived benefit magnitude (F7,360 = 1.97, P-value = 0.059) and benefit likelihood (F7,360 = 1.90, P-value = 0.069), perceived comparative efficacy relative to other prescription drugs (F7,360 = 1.22, P-value = 0.293) and relative to Mytrozen (F7,360 = 1.36, P-value = 0.222), perceived severity of side effect (F7,360 = 0.49, P-value = 0.842), and likelihood of side effect (F7,360 = 0.67, P-value = 0.694); and perceived comparative risk relative to other prescription drugs (F2,140 = 0.20, P-value = 0.820) and relative to Mytrozen (controlling for age and experience post residency [F2,143 = 0.53, P-value = 0.588]).
Attitudes Toward the Biosimilar and Intentions
Table 4 shows mean scores attitude and intention measures by biosimilar definition characteristic. For consumers, results of omnibus tests indicated no significant effect of biosimilar identification, biosimilar definition, or named reference product on attitudes toward the biosimilar product (controlling for health literacy [F7,370 = 1.09, P-value = 0.366]) or intention to take the drug (controlling for health literacy [F7,370 = 1.37, P-value = 0.216]) or switch to a biosimilar drug (controlling for health literacy [F7,370 = 1.02, P-value = 0.418]).
HCP Perceptions, Attitudes and Intentions by Biosimilar Disclosure Characteristics
For HCPs, we found an effect of biosimilar identification on attitudes toward the biosimilar, such that HCPs who saw any disclosure (M = 4.18, SE = 0.15) reported more positive attitudes than the no disclosure definition condition (M = 3.80, SE = 0.16, t-value = 2.29, P-value = 0.023, ƞ2 = 0.01). Findings also showed an effect of biosimilar definition on attitudes, such that HCP participants exposed to the definition that included all three statements about biosimilars reported more positive attitudes toward biosimilars (M = 4.23, SE = 0.15), compared with participants exposed to the definition that only indicated the drug was a biosimilar (M = 3.83, SE =0.15, t-value = 2.47, P-value = 0.014, ƞ2 = 0.02) and the control (M = 3.80, SE = 0.16, t-value = 2.61, P-value = 0.009, ƞ2 = 0.02). We did not observe a significant effect of naming the reference product on attitude toward the drug (t-value = 0.40, P-value = 0.691).
For HCPs, there was a main effect of biosimilar identification on intention to prescribe such that those who saw any disclosure reported higher intentions (M = 3.65, SE = 0.19) compared with the control (t-value = 2.14, P-value = 0.033, ƞ2 = 0.01). Further, findings showed a main effect of biosimilar definition such that those exposed to the definition that included all three statements about biosimilars reported higher intentions to prescribe (M = 3.76, SE = 0.18) compared with the control (M = 3.21, SE = 0.19, t-value = 2.64, P-value = 0.009, ƞ2 = 0.02) and the condition that identified Kesterin as a biosimilar only with no further definition (M = 3.00, SE = 0.19, t-value = 3.75, P-value <0.001, ƞ2 = 0.04). We did not observe a significant effect of the named reference product on intentions to prescribe the drug (t-value = 0.07, P-value = 0.946).
Perceptions around interchangeability of a biosimilar with a reference product (F5,273 = 0.73, P-value = 0.604) did not vary by disclosure condition.
Biosimilar Disclosure Preference
Tables 5 and 6 show preferences for the seven biosimilar disclosure definitions for consumers and HCPs, respectively. Consumers tended to prefer the definition that included all three statements about biosimilars, but did not name the reference product Mytrozen, regardless of whether they were assigned to that experimental condition (69.9% and 69.0%): KESTERIN is a biosimilar. This biosimilar is a safe and effective medication and provides the same treatment benefits as an FDA-approved original biologic. Biosimilars are made from the same types of sources as the FDA-approved original biologic. KESTERIN is given the same way and has the same strength and dosage as the FDA-approved original biologic. The least preferred biosimilar disclosure stated Kesterin is a biosimilar but did not provide any further information (8.7% and 8.8%).
Consumer Preferences for Biosimilar Disclosures
HCP Preferences for Biosimilar Disclosures
HCPs tended to prefer the definition that included all three statements about biosimilars and also named the referred drug Mytrozen, regardless of whether they were assigned to that experimental condition (73.2% and 72.8%): KESTERIN is a biosimilar. This biosimilar is a biological product that is highly similar to and has no clinically meaningful differences from MYTROZEN, an existing FDA-approved reference product. This biosimilar is made from the same types of sources as MYTROZEN. KESTERIN has the same route of administration, dosage form, and strength as MYTROZEN. The least preferred biosimilar disclosure stated Kesterin is a biosimilar but did not provide any further information (12.3% and 8.5%).
Discussion
Research has suggested that HCPs have certain knowledge gaps related to biosimilar products, particularly in regard to their efficacy and safety relative to reference biologics.5,7–10 Consumers similarly lack awareness of and knowledge about biosimilar products. The current study aimed to understand how providing different levels of information about a biosimilar in the form of a disclosure statement influenced understanding and perceptions about these products. We explored this aim by experimentally testing disclosure language for a fictitious biosimilar Kesterin on a mock branded prescription drug website, as web sites are a common resource when seeking information about prescription drugs.22 Our fictitious advertisement was designed to resemble what HCPs and consumers might actually see in the marketplace.
Consumers and sometimes HCPs had less than optimal understanding of biosimilars, even when provided with a definition and asked questions about what they just read. About half of consumers and two-thirds of HCPs correctly understood that a biosimilar and the reference product are comparable in terms of safety and efficacy, that they are made from the same types of sources, and that they have the same dosage and administration. These knowledge gaps signal an opportunity for further evidence-based education around biosimilars and the need for carefully defining these products in promotional communications or other health communications. Although our study was not designed to explore this concept more deeply, the finding that sizeable proportions of respondents did not correctly identify basic characteristics of biosimilars is potentially concerning, particularly among the HCP group. Further, HCPs were in general agreement with a statement that biosimilars could be used “interchangeably” with the original biologic. This general agreement could signal some misunderstanding about what is required for a regulatory designation of “interchangeability.”
Prior research has suggested that consumers and HCPs sometimes perceive that biosimilars are less efficacious and have more side effects than other products.10–13 Consumers and HCPs in our study, in general, had neutral perceptions of biosimilar benefits, efficacy relative to other prescription drugs, and safety (scores for these outcomes hovered around the midpoint). These neutral perceptions may signal some uncertainty around biosimilars. In addition, these perceptions did not vary across the different disclosure conditions, including a control condition with no disclosure, suggesting that perceptions around biosimilars appeared to be fixed, regardless of the information presented.
Manipulation of the biosimilar disclosure had few effects on outcomes, with some exceptions. HCPs expressed more positive attitudes toward the biosimilar drug when provided with the disclosures with the most information. This finding suggests that the expanded definitions were perceived as potentially useful. We further found that both consumers and HCPs said that they preferred the expanded definitions compared with those that provided less information. HCPs tended to prefer seeing the name of the reference product Mytrozen rather than “an FDA-approved reference product.” Despite this stated preference, naming the reference product had no effect on study outcomes for either HCPs or consumers. This lack of finding may be due, in part, to the fictitious naming of the reference product as Mytrozen, which would not have been a recognizable product to either HCPs or consumers. Although we instructed participants that drug names were changed for the purposes of the study, it is plausible that perceptions may have varied had the reference product been a common name that respondents would have recognized. We also found that HCPs reported higher intentions to (hypothetically) prescribe the biosimilar when presented with the expanded definitions. While it is unclear how these intentions would translate to actual prescribing, the finding provides further evidence for the value of providing basic information on the products’ similarity to the reference product in terms of safety, efficacy, sources, dosage, and administration.
Limitations
This study has some limitations. Our study was intended to explore perceptions around biosimilars among general consumers of prescription drugs rather than within one specific medical condition. Research with populations who rely most heavily on these products, such as those receiving treatment for cancer or autoimmune medical conditions, could provide a more comprehensive picture of attitudes and understanding of biosimilar product information. Similarly, our HCP sample included only primary care providers. While about three-fourths of the HCP sample reported some familiarity with biosimilars, it is plausible that medical specialist who prescribe biosimilars most often may have responded differently. Replication of findings in more specialized medical populations may be warranted. It is also unclear if participants’ neutral perceptions and attitudes reflected thoughts about the biosimilar product itself or the stimuli, where we presented a static webpage promotion with a blurred background where only the disclosure definition language was visible. Despite providing survey instructions explaining that much of the page would be blurred and to focus on the language (disclosure) that was visible, some study participants commented in the survey that the study stimuli did not provide enough information to form an opinion.
Conclusion
Our study findings signal that opportunities exist for further education around biosimilar products. Despite providing study participants with definitions about biosimilars, sizeable proportions of both the consumer and HCP groups demonstrated some misunderstanding or uncertainty around these products. Further message testing and education, specifically among populations who may use or prescribe these products more regularly, could help pinpoint specific areas for improvement and education.
Notes
This article was externally peer reviewed.
Funding: Financial support for this study was provided entirely by a contract with the U.S. Food and Drug Administration. The following authors are employed by the sponsor: Kathryn J. Aikin and Amie C. O’Donoghue.
Conflict of interest: The authors have no conflicts of interests to disclose.
To see this article online, please go to: http://jabfm.org/content/38/1/94.full.
- Received for publication April 10, 2024.
- Revision received August 9, 2024.
- Accepted for publication August 16, 2024.